Published online Jun 28, 2015. doi: 10.4254/wjh.v7.i12.1694
Peer-review started: November 29, 2014
First decision: January 8, 2015
Revised: January 20, 2015
Accepted: May 26, 2015
Article in press: May 27, 2015
Published online: June 28, 2015
Processing time: 213 Days and 1.2 Hours
AIM: To evaluate the downstaging rates in hepatitis C virus-patients with hepatocellular carcinoma (HCC), treated with degradable starch microspheres transcatheter arterial chemoembolization (DSM-TACE), to reach new-Milan-criteria (nMC) for transplantation.
METHODS: This study was approved by the Ethics Committee of our institution. From September 2013 to March 2014 eight patients (5 men and 3 women) with liver cirrhosis and multinodular HCC, that did not meet nMC at baseline, were enrolled in this study. Patients who received any other type of treatment such as termal ablation or percutaneous ethanol injection were excluded. DSM-TACE was performed in all patients using EmboCept® S and doxorubicin. Baseline and follow-up computed tomography or magnetic resonance imaging was assessed measuring the longest enhancing axial dimension of each tumor according to the modified Response Evaluation Criteria In Solid Tumors measurements, and medical records were reviewed.
RESULTS: DSM-TACE was successfully performed in all patients without major complication. We treated 35 lesions (mean 4.3 per patient). Six of eight patients (75%) had their HCC downstaged to meet nMC. Every patient whose disease was downstaged eventually underwent transplantation. The six patients who received transplant were still living at the time of this writing, without recurrence of HCC. Baseline age (P = 0.25), Model for End-stage Liver Disease score (P = 0. 77), and α-fetoprotein level (P = 1.00) were similar between patients with and without downstaged HCC.
CONCLUSION: DSM-TACE represents a safely and effective treatment option with similar safety and efficacy of conventional chemoembolization and could be successfully performed also for downstaging disease in patients without nMC, allowing them to reach liver transplantation.
Core tip: Liver transplantation (LT) is the standard of care for select patients with hepatocellular carcinoma (HCC) and cirrhosis and recently more transplant centers use the new Milan criteria to assess the candidacy of HCC patients for LT. This manuscript reports a preliminary experience on the HCC treatment in liver transplant candidates without new-Milan-criteria, using a new technique of transcatheter arterial chemoembolization with degradable starch microspheres transcatheter arterial chemoembolization (DSM-TACE). Providing a temporary embolization DSM-TACE allows to treat more patients with an impaired liver function reducing toxicity due to standard arterial embolization. Moreover good down-staging rates after repeated DSM-TACE were successfully achieved.
- Citation: Orlacchio A, Chegai F, Merolla S, Francioso S, Giudice CD, Angelico M, Tisone G, Simonetti G. Downstaging disease in patients with hepatocellular carcinoma outside up-to-seven criteria: Strategies using degradable starch microspheres transcatheter arterial chemo-embolization. World J Hepatol 2015; 7(12): 1694-1700
- URL: https://www.wjgnet.com/1948-5182/full/v7/i12/1694.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i12.1694
Currently, trans-arterial chemoembolization (TACE) represents the most accepted and widely used treatment for large or multinodular hepatocellular carcinoma (HCC), notably in patients with a relatively preserved liver function without vascular invasion and/or extra-hepatic spread[1].
HCC ranks as the third most common cause of cancer-related death and is the sixth most common form of cancer worldwide[2].
The purpose of TACE is to reach high and lasting concentrations of chemotherapeutic drugs in the tumor site in order to increase their uptake by neoplastic cells, reducing at the meantime systemic side effects.
Nevertheless, liver transplantation (LT) remains the ideal treatment for small HCC resulting from chronic liver disease. Given the difference between donor organ availability and need, rigorous inclusion criteria, named the Milan criteria, have been originally adopted to warrant tumor free survival after LT. They was defined as having a single tumor 5 cm or less in diameter in patients with single HCC, or up to 3 separate lesions < 3 cm and without microscopic vascular invasion or extra-hepatic spread[3].
The excessive intransigence of those criteria has been a matter of debate, with some groups questioning their restrictive settings[4,5]. Afterwards, the tendency of the Milan group was to make the previous criteria less stringent, so were developed new ones called the up-to-seven criteria [new-Milan-criteria (nMC)]: HCCs with seven as the sum of the size of the largest tumor (in cm) and the number of tumors[6]. Several studies have then evaluated their reliability and usefulness in assessing the possible candidates for LT among patients with HCC[7,8]. Nevertheless, albeit have been analyzed all over the world, up-to-seven criteria have not been as widely accepted as the Milan criteria.
In prospective studies on selected patients with HCC, loco-regional treatments has been shown both to downstage the disease and to confer acceptable disease free survival after liver trasplantation. Several locoregional treatment for downstaging may be used[9,10]. Green et al[10] using doxorubicin-eluting bead transarterial chemoembolization showed that a treatment with drug eluting beads (DEB) has a high likelihood (77%) of downstaging the disease so that it can meet Milan criteria.
Nevertheless, by interrupting blood flow to the tumor, TACE induces necrosis at the site of disease but it may create conditions that permit or even encourage angiogenesis[11].
A recent study performed by Pieper et al[12] in a swine model demonstrated that using degradable starch microspheres (DSMs) a short term embolization can be achieved (30 min), without significant hystological differences in damage between treated or untreated liver. Thus, using DSMs it is possible to avoid the paradoxical angiogenetic effect due to ischemia[13], emphasizing the effects of arterial chemotherapy.
As reported by Furuse et al[14] in their work, DSM-TACE should not be employed as a single session therapy but needs to be performed at least three times until there was evidence of disease progression or unacceptable toxicity.
Anyhow this procedure is well tolerated by the patients and it could be safe also in those with a severe hepatic disease, even with a Child-Pugh score C.
The aim of our work is to assess downstaging rates in patients with HCC using DSM TACE and doxorubicin, in order to meet new Milan criteria and to create conditions allowing LT.
The herein described study was approved by the Ethics Committee of our institution. The Child-Pugh score, the Model for End Stage Liver Disease (MELD score), radiological and pathological tumor classification according to nMC, demographics (age, sex), etiology of cirrhosis, laboratory tests, imaging studies and pathology reports were recorded for each patient.
Exclusion criteria were an active peptic ulcer, unmanageable ascites or pleural effusion, hepatic encephalopathy, or any other preexisting medical condition of sufficient severity to prevent full compliance with the study. Moreover, patients who received any other type of treatment such as thermoablative treatment, or percutaneous ethanol injection were excluded.
All patients in our study were selected during the follow-up for liver cirrhosis and the opportunity of locoregional ablative treatment was carefully evaluated by a multidisciplinary team of hepatologists, surgeons, radiologists and interventional radiologists[15].
We have selected patients with a multinodular HCC that did not meet, at baseline, Milan criteria. The diagnosis of HCC was set according to the guidelines of the European Association for the Study of the Liver[16].
All patients underwent, within two weeks before treatment, a dynamic multislice computed tomography (CT) (Light Speed 64 CT, GE Medical Systems, Milwaukee, United States) (Figure 1).
A dynamic CT evaluation following the DSM-TACE treatment was performed to assess whether HCC patients were downstaged to meet the nMC.
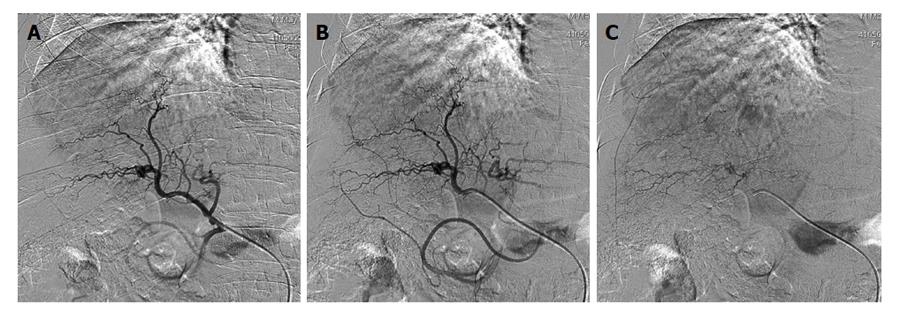
Under local anesthesia the right common femoral artery was punctured using the Seldinger approach and a 4-French (4-Fr) sheath introducer was placed to secure the access site. Selective hepatic angiography was then performed with a 4-Fr catheter. The tip of the catheter was placed in the proper hepatic artery or right and/or left hepatic artery to inject embolic agents (Figure 2).
A combination of DSM, at a concentration of 225 mg per 6 mL of contrast medium, and doxorubicin at a dose of 50 mg/m2, was injected until embolization of peripheral hepatic arteries was confirmed; the aforementioned procedure was repeated for three times every 4 to 6 wk.
All patients were scanned by contrast-enhanced CT (ceCT), at the baseline, within two weeks before the first DSM-TACE. Thirty days after each DSM-TACE, patients were re-assessed by ceCT.
To assess any changes in the size of the nodule and/or appearance of new lesions, multidetector ceCT scan was obtained with triphasic technique (30, 65 and 180 s) after intravenous administration of 125-175 mL of iodinate contrast medium (350 mg iodine/mL) depending on the body mass index of the patient, followed by administration of saline solution (20-30 mL) using an automatic injector at flow rates of 3-4 mL/s.
CT was performed with the following parameters: rotation time of 0.6 s; 2.5 to 5 mm thick sections with the possibility of back-reconstructions up to 0.6 mm; automatic milliamperage (mA) (min 300 mA, max 450 mA), 120 kV (Figure 2).
Therapeutic response was determined on a lesion-by-lesion basis by evaluating morphological and vascularization changes in tumor between baseline and subsequent ratings based on ceCT.
The treatment response was evaluated according to modified Response Evaluation Criteria in Solid Tumors criteria[17]. Based on CT results, tumor response to treatment, were defined complete [complete response (CR)] when there was disappearance of all signs of the lesion and no pathological enhancement was detectable on the edges of the area treated and partial or incomplete [partial response (PR)] in case of a limited destruction of the lesion, with at least a 30% reduction in volume. A lesion not presenting such dimensional decrease or increase was considered stable disease (SD).
A descriptive statistics for baseline demographics and staging criteria were calculated.
Continuous variables were reported as the mean ± SD or the median (25%-75% interquartile range), depending on the variable distribution whereas categorical variables were reported as numbers and percentages.
Recurrence-free survival was calculated from the day of the transplantation to the first evidence of tumor relapse during follow-up or, in patients without recurrence, to the most recent follow-up visit. Follow-up of those patients who died without evidence of recurrence was censored at the time of death.
From September 2013 to March 2014 eight patients (5 men and 3 women) with liver cirrhosis and multinodular HCC were enrolled in this study.
Demographics and clinical characteristic are reported in Table 1.
| Characteristics | |
| Gender (male), n | 5 |
| Mean age (yr) ± SD | 59 ± 6 |
| Mean aspartate aminotransferase levels (IU/L) ± SD | 68.8 ± 53 |
| Mean alanine transaminase levels (IU/L) ± SD | 62.5 ± 65 |
| Mean gamma-glutamyl-transpeptidase levels (IU/L) ± SD | 156 ± 211 |
| Mean creatinine levels (mg/dL) | 0.86 ± 0.25 |
| Mean international normalized ratio | 1.2 ± 0.1 |
| Mean platelet levels (103/μL) ± SD | 975000 ± 57000 |
| Mean alfa fetoprotein (ng/mL) ± SD | 14.9 ± 21.4 |
The age of patients ranged from 56-64 years. Four patients were in Child-Pugh class B and four patients were in Child-Pugh class C. All of them had hepatitis C virus (HCV)-related cirrhosis, 2 patients had a coinfection with HBV and one had a combined HCV and alcohol related cirrhosis.
The α-fetoprotein (AFP) levels, registered before the procedure, were on average 14.9 ± 21.4 ng/mL.
A total of 35 HCC nodules were treated, 25 of which were located in the right lobe (segments V-VIII) and 10 in the left lobe (segments I-IV). No major complications were observed after DSM-TACE. The post-embolization syndrome (transient fever, abdominal pain, nausea) was the most common complication following chemoembolization; all side effects were successfully treated with medical therapy.
Six patients met the nMC and had downstaging of their disease (Figure 3). Two patients were considered non-downstaged. Baseline age (P = 0.25), Model for End-stage Liver Disease score (P = 0.77), and AFP level (P = 1.00) were similar between patients with and without downstaged HCC.
Complete necrosis of tumor with no evidence of viable tumor on pathologic examination was observed in 4 patients. After the treatments 18/35 lesions were classified as CR, 12 as PR and 5 as SD. Microscopically, in all lesions classified as CR, tumor necrosis was mixed, both colliquative and coagulative. The median time spent on the waiting list was 3.3 mo in patients downstaged with DSM-TACE. All six patients downstaged were successfully transplanted and after three months from LT did not experience tumor recurrence.
One of the main drawbacks of LT in patients with HCC is the downtime on the waiting list while the risk of tumor progression increases, resulting in a cumulative probability of dropout from the waiting list of 7.2% for a 6-mo waiting time, which rises to 37.8% and 55.1% for 12 and 18 mo of waiting time, respectively[18].
Thirteen years after the Milan criteria were developed, the Mazzaferro group developed more indulgent criteria called the up-to-seven criteria (nMC). In their study, the 5-year overall survival rate for Milan group patients was 73.3% and overall survival rate was 71.2%[5].
Afterwards, numerous studies have appraised the effectiveness of adopting the up-to-seven criteria as inclusion criteria for HCC LT. Similar post LT survival rates among patients were demonstrated in a study by de Ataide et al[19]. They directly compared the long-term outcomes of an up-to-seven criteria group, whose survival rates of 87.7%, 74.5% and 65.3% at 1, 3, and 5 years were similar to those in patients meeting the Milan criteria.
In our preliminary experience we achieved a downstaging disease within the nMC in 75% of patient treated with DSM-TACE. Moreover these patients were successfully transplanted without any complication.
In a study by Green et al[10] with DEB-TACE in patient with T3N0M0 HCC, were obtained survival rates quite comparable with our results with an high likelihood (77%) of downstaging the disease to meet Milan criteria.
In another recent work by Nicolini et al[20] on recurrence-free survival after LT in patients with HCC, were assessed the possible effect of two different types of preoperative TACE. Additionally, the effects of TACE on tumor were histologically analyzed. The authors used conventional Lipiodol TACE and DEB-TACE gaining better result with the latter treatment.
DEB-TACE could effectively promote tumor necrosis and improving recurrence-free survival after LT in HCC. In the authors’ opinion the best result carried out with DEB TACE was mostly due to the increase of intra-tumor drug delivery. Indeed there are 3 main pharmacokinetic advantages associated with DEB-TACE: a long lasting elution of the drug, an higher concentration distributed locally into the tumor and a lower systemic exposure to the drug in comparison with conventional-TACE[21].
DSM were developed to avoid a permanent occlusion of blood flow to the tumor, a condition which could permit or even encourage angiogenesis.
The microspheres are 45 ± 7 × 10-6 m in diameter and descend until the arteriole/capillary level, where they lodge[22]. The duration of occlusion in the hepatic arteries by DSM may range between 30 and 80 min. An anticancer drug coadministered with DSM is selectively trapped with DSM in small arteries, and is concentrated in areas of liver tumor. Consequently, the activity of the drug is expected to increase in effectiveness and durability. Thus, in light of the expected reduction in toxicity levels and adverse events, it could be used safety in patients with Child C or a high MELD score.
In this respect, Niessen et al[23] shown that patients with unresectable intermediate-stage HCC had a lower increase in aminotransferase when they were treated with DSM-TACE compared to patients treated with doxorubicin and ethidiol TACE. In our experience the procedure with DSM-TACE was well tolerated by patients and no major complications were seen.
However, the present study results have to be viewed with caution because this is a preliminary experienced with a relative new type of chemoembolization agent and because of the small number of patients and the relative short time of follow-up after the LT.
These concerns were also expressed by Sotiropoulos et al[24] who, in a letter to Mazzaferro et al[6], stated that although the up-to-seven criteria are based on objective tumor characteristics such as tumor size, tumor number and microvascular invasion, these aspects represent pathology findings and not preoperative objective tumor characteristics. Hence, they conclude that the up-to-seven criteria are illusive and not applicable in clinical practice[24].
Anyhow, in our experience, DSM-TACE represents an effective treatment option with a similar safety and efficacy as a conventional TACE (Lipiodol or DEB), and could be safety and successfully used in patients without nMC for downstaging disease.
The paper trys to evaluate the possible down-staging rate using degradable starch microspheres transcatheter arterial chemoembolization (DSM-TACE) in hepatocellular carcinoma (HCC) patients without new-Milan-criteria (nMC). Liver transplantation remains the ideal treatment for small HCC and several locoregional treatment for downstaging may be used when patients were out from transplant criteria. DSMs are new materials for embolization which permit to obtain a good results also in patients with an unpaired liver function.
This new technique permits to dowsntage HCC patients without nMC saftely and with a good results in term of tumor response and tolerability.
This is the first study where DSM TACE was used to obtain a down staging disease in patients with multi-nodular HCC. Previous study evaluated the efficacy of conventional TACE and drug eluting beads-TACE.
DSM-TACE represents an effective simple treatment option with a similar safety and efficacy as a conventional TACE. Transient occlusion of tumor feeding arteries permits to obtain good results in terms of tumor response without significant worsening liver function. It can be used safety in multifocal and multi-nodular HCC even when HCC has a bilobar localization.
DSM: Degradable starch microspheres. The starch microspheres consist of a three-dimensional, cross linked hydrophilic starch matrix, which swells heavily in a water suspension environment (the specific diameter refers to its swollen state) and are completely degradable by amylase.
It is very interesting research work.
P- Reviewer: Lorenzo-Zuniga V, Ozenirler S, Pan WS S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6545] [Article Influence: 467.5] [Reference Citation Analysis (1)] |
| 2. | Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10002] [Cited by in RCA: 10438] [Article Influence: 695.9] [Reference Citation Analysis (0)] |
| 3. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5280] [Article Influence: 182.1] [Reference Citation Analysis (0)] |
| 4. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1682] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 5. | Marsh JW, Dvorchik I. Liver organ allocation for hepatocellular carcinoma: are we sure? Liver Transpl. 2003;9:693-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [PubMed] |
| 7. | D’Amico F, Schwartz M, Vitale A, Tabrizian P, Roayaie S, Thung S, Guido M, del Rio Martin J, Schiano T, Cillo U. Predicting recurrence after liver transplantation in patients with hepatocellular carcinoma exceeding the up-to-seven criteria. Liver Transpl. 2009;15:1278-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Chan SC, Fan ST, Chok KS, Cheung TT, Chan AC, Fung JY, Poon RT, Lo CM. Survival advantage of primary liver transplantation for hepatocellular carcinoma within the up-to-7 criteria with microvascular invasion. Hepatol Int. 2012;6:646-656. [PubMed] |
| 9. | Yu CY, Ou HY, Huang TL, Chen TY, Tsang LL, Chen CL, Cheng YF. Hepatocellular carcinoma downstaging in liver transplantation. Transplant Proc. 2012;44:412-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 10. | Green TJ, Rochon PJ, Chang S, Ray CE, Winston H, Ruef R, Kreidler SM, Glueck DH, Shulman BC, Brown AC. Downstaging disease in patients with hepatocellular carcinoma outside of Milan criteria: strategies using drug-eluting bead chemoembolization. J Vasc Interv Radiol. 2013;24:1613-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Lencioni R. Management of hepatocellular carcinoma with transarterial chemoembolization in the era of systemic targeted therapy. Crit Rev Oncol Hematol. 2012;83:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Pieper CC, Meyer C, Vollmar B, Hauenstein K, Schild HH, Wilhelm KE. Temporary arterial embolization of liver parenchyma with degradable starch microspheres (EmboCept®S) in a swine model. Cardiovasc Intervent Radiol. 2015;38:435-441. [PubMed] |
| 13. | Lindell B, Aronsen KF, Rothman U. Repeated arterial embolization of rat livers by degradable microspheres. Eur Surg Res. 1977;9:347-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Furuse J, Ishii H, Satake M, Onaya H, Nose H, Mikami S, Sakai H, Mera K, Maru Y, Yoshino M. Pilot study of transcatheter arterial chemoembolization with degradable starch microspheres in patients with hepatocellular carcinoma. Am J Clin Oncol. 2003;26:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 15. | Gish RG, Lencioni R, Di Bisceglie AM, Raoul JL, Mazzaferro V. Role of the multidisciplinary team in the diagnosis and treatment of hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2012;6:173-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3234] [Article Influence: 134.8] [Reference Citation Analysis (0)] |
| 17. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2583] [Cited by in RCA: 3260] [Article Influence: 217.3] [Reference Citation Analysis (36)] |
| 18. | Yao FY, Bass NM, Nikolai B, Merriman R, Davern TJ, Kerlan R, Ascher NL, Roberts JP. A follow-up analysis of the pattern and predictors of dropout from the waiting list for liver transplantation in patients with hepatocellular carcinoma: implications for the current organ allocation policy. Liver Transpl. 2003;9:684-692. [PubMed] |
| 19. | de Ataide EC, Garcia M, Mattosinho TJ, Almeida JR, Escanhoela CA, Boin IF. Predicting survival after liver transplantation using up-to-seven criteria in patients with hepatocellular carcinoma. Transplant Proc. 2012;44:2438-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Nicolini D, Svegliati-Baroni G, Candelari R, Mincarelli C, Mandolesi A, Bearzi I, Mocchegiani F, Vecchi A, Montalti R, Benedetti A. Doxorubicin-eluting bead vs conventional transcatheter arterial chemoembolization for hepatocellular carcinoma before liver transplantation. World J Gastroenterol. 2013;19:5622-5632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, Montañá X, Llovet JM. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 715] [Article Influence: 39.7] [Reference Citation Analysis (1)] |
| 22. | Sigurdson ER, Ridge JA, Daly JM. Intra-arterial infusion of doxorubicin with degradable starch microspheres. Improvement of hepatic tumor drug uptake. Arch Surg. 1986;121:1277-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Niessen C, Unterpaintner E, Goessmann H, Schlitt HJ, Mueller-Schilling M, Wohlgemuth WA, Stroszczynski C, Wiggermann P. Degradable starch microspheres versus ethiodol and doxorubicin in transarterial chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol. 2014;25:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Sotiropoulos GC, Molmenti EP, Lang H. Milan criteria, up-to-seven criteria, and the illusion of a rescue package for patients with liver cancer. Lancet Oncol. 2009;10:207-208; author reply 208-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |